(This post is by Mariëlle van Toor, on Twitter as @mlvantoor)
Most of the stories on this blog somehow relate to birds (particularly ducks) and pathogens (most often avian influenza viruses, or AIV). Throughout the years, we have established that ducks are quite good at transporting AIV during their daily movements, and even migration. But viruses are not the only thing that ducks are able transport! Specialist wetland plants, which inhabit discrete habitats that are often not connected by waterways, have usually very little opportunity for (long-distance) dispersal that would allow them to colonise new wetlands. Dabbling ducks such as the mallards forage on all kinds of seeds, which they crush in a specialised organ called gizzard to make them easier to digest. However, seeds often escape from the gizzard unharmed. These seeds will be excreted by the ducks rather than digested, and can grow into mature plants. By transporting the seeds of wetland plants in this way, ducks could fill an ecologically important role.
But just how much could migratory ducks such as mallards contribute to seed dispersal? This was the question that our collaborator Erik Kleyheeg, then a PostDoc at the Max Planck Institute for Ornithology, approached us with. Erik (now also known as the “Teal man”) had already accumulated a wealth of knowledge on mallard seed dispersal during his PhD, and had collected spring migratory tracks of mallards wintering at Lake Constance. Combined with the extensive ring recovery data set from the same population of mallards, the duration of seed passage through the gut, and the models we previously applied for our virus dispersal paper (see post here), we developed our “comprehensive mallard seed dispersal model” that is now published in Frontiers in Ecology and Evolution.
But what does comprehensive mean in terms of mallard seed dispersal? For that we need to know what previous attempts at modelling seed dispersal by mallards during migration looked like. Often, previous studies have estimated seed dispersal distances during bird migration by multiplying distributions of gut retention time with the flight speed of birds. But neither do ducks fly in a straight line, nor do they fly uninterruptedly between their wintering and breeding areas. We thus need to account for staging behaviour and specifics of the migratory flight, and consider dispersal during the different stages of migration.

The black line shows the curve describing the retention of seeds in a mallard’s gut. Most seeds will be excreted soon after ingestion (depending on seed size), but the long tail could enable ducks to transport seeds over long distances both during migratory legs and staging periods.
Our model includes both seed dispersal during the actual migratory flight, and during the time that ducks are staying at stopover sites.
Another important aspect is that we need to account for when individuals stop foraging. Do they eat seeds right up until the start of a migratory flight, or do individuals fast prior to migration to avoid carrying around any extra weight? As the answer to this question is not satisfactorily resolved yet, we decided that our model should be able to account for fasting time. For our paper, we considered three scenarios – no fasting, short fasting (1 hour prior to migration), and long fasting (12 hours prior to migration) – to understand how fasting would affect seed dispersal. As soon as we better understand the behaviour of pre-migratory fasting in ducks, however, we can feed any number into the model for more specific (and realistic) predictions.

The duration of pre-migratory fasting affects potential dispersal distances quite substantially. Above you can see how short and long retention times (for small and large seeds, respectively) are affected by fasting duration.
Finally, all seed dispersal is to no end if seeds are not transported to habitat for suitable for germination. For wetland plants, that obviously means transport to other wetlands. While all other components of the seed dispersal kernel derived from our model should be transferable to other mallard populations, the availability of wetlands along the migratory corridor were specific to this population. We calculated the probability of dispersed seeds to end up in wetland habitat for both staging mallards, which can be found in wetlands most of the time, and for migratory individuals, for which we used the Global Lakes and Wetlands Database (GLWD). Our summary map for wetland availability already shows that it is not very likely for a seed dispersed by an actively migrating mallard to be deposited in a wetland:

Most migratory mallards wintering at Lake Constance migrate along a N-E corridor. The highest concentration of wetland area lie towards the East and North East.
All this taken together, the question is: How good are mallards as seed dispersers? You won’t like the answer: it depends! Theoretically, mallards are amazing at transporting seeds – if they don’t fast before starting migration. If mallards foraged right up until migration, they could transport a large part of the swallowed seeds over hundreds of kilometers, and many of them would end up in suitable habitat during the first stopover period. But the duration of pre-migratory fasting hugely influences dispersal distances, and in the 12 hour scenario, many seeds were already excreted prior to migration. But even then, some seeds can be transported over exceptional distances.

These are the final seed dispersal kernels predicted from our model, shown for short and long retention times, and three different scenarios of pre-migratory fasting. The upper row shows the general probability of seeds being dispersed over distances up to 950 km (the general prediction), whereas the lower row shows the probability of seeds being dispersed into suitable habitat (the prediction specific to Lake Constance mallards).
In conclusion, migratory ducks such as mallards likely play an important role for the short- and long-distance dispersal of wetland plants, both during periods of migration and residency. But, as always, there are more questions to be answered before we can say for sure.
Link to the paper:







 There’s no business, like duck business
There’s no business, like duck business


 For a pathogen to survive it has to find new hosts to infect. This may sound simple, but if you consider the entangled mesh that is the biology of a host species you realize that there are plenty of ways that things can go wrong, stopping the chain of transmission. First of all, the harm the pathogen incurs on its host – the virulence – needs to be balanced between being too low – the infection will be cleared before any transmission opportunities have occurred – or too high, so that it causes the demise of the host before transmission can take place. Second, the pathogens must overcome the hurdles of moving from one host to the next, be it in water, air or through the bite of an arthropod vector. And third, it has to overcome the fact that most hosts are not sedentary, but move varying distances in response to changes in the environment they inhabit. Finally, it needs to be able infect the new host and evade the immune system to establish infection. Not an easy feat, but something that is happening all the time in the world of viruses, bacteria, fungi, parasites and their hosts.
For a pathogen to survive it has to find new hosts to infect. This may sound simple, but if you consider the entangled mesh that is the biology of a host species you realize that there are plenty of ways that things can go wrong, stopping the chain of transmission. First of all, the harm the pathogen incurs on its host – the virulence – needs to be balanced between being too low – the infection will be cleared before any transmission opportunities have occurred – or too high, so that it causes the demise of the host before transmission can take place. Second, the pathogens must overcome the hurdles of moving from one host to the next, be it in water, air or through the bite of an arthropod vector. And third, it has to overcome the fact that most hosts are not sedentary, but move varying distances in response to changes in the environment they inhabit. Finally, it needs to be able infect the new host and evade the immune system to establish infection. Not an easy feat, but something that is happening all the time in the world of viruses, bacteria, fungi, parasites and their hosts.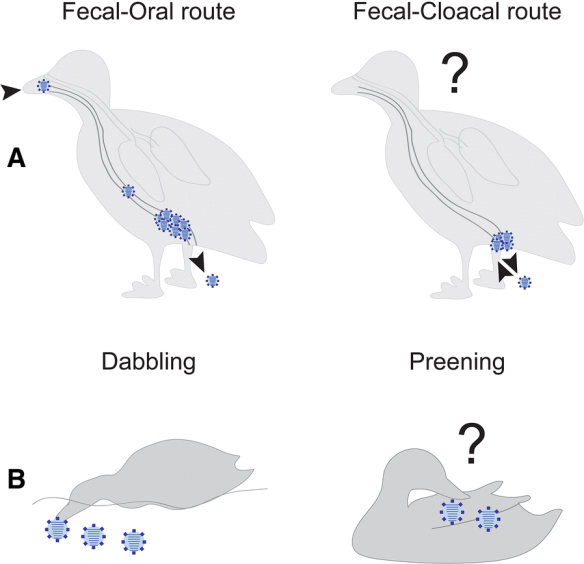











 The life of teal is a life on the wing. It is the smallest dabbling duck in the Boreal zone, but in terms of migration it covers huge distances from breeding waters to the non-breeding areas far, far away. The same bird can spend time in an oligotrophic lake in Russia during breeding, a brackish Baltic Sea lagoon or a tidal mudflat in the Atlantic coast during autumn, and a rich river delta in the Mediterranean in winter – and some individuals even straddle over into North Africa for a dabble. It takes a lot of adaptability to switch between such vastly different habitats, and at the same time avoid becoming dinner of arial predators and human hunters.
The life of teal is a life on the wing. It is the smallest dabbling duck in the Boreal zone, but in terms of migration it covers huge distances from breeding waters to the non-breeding areas far, far away. The same bird can spend time in an oligotrophic lake in Russia during breeding, a brackish Baltic Sea lagoon or a tidal mudflat in the Atlantic coast during autumn, and a rich river delta in the Mediterranean in winter – and some individuals even straddle over into North Africa for a dabble. It takes a lot of adaptability to switch between such vastly different habitats, and at the same time avoid becoming dinner of arial predators and human hunters.